Emulsion-Based Techniques for development of Pharmaceutical Nanoparticle Dosage Forms
- Moral Randeria

- Aug 1, 2025
- 7 min read
Updated: Sep 16, 2025
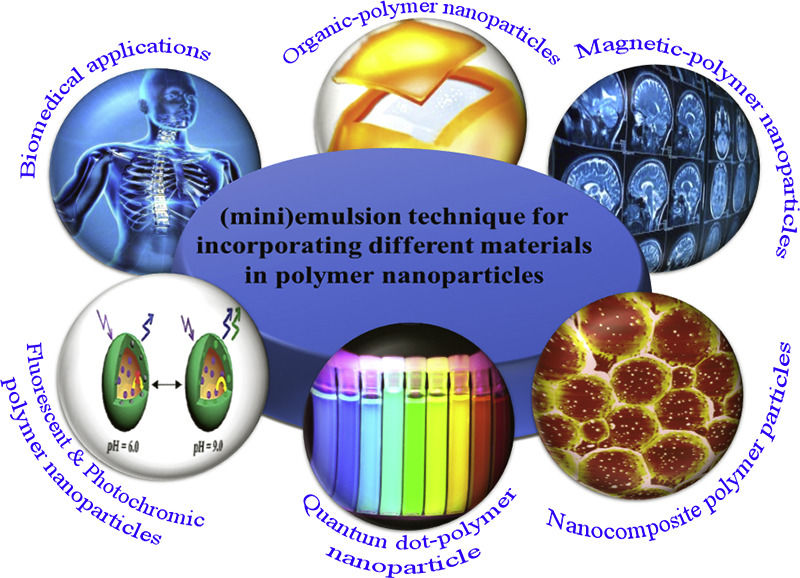
Excipients, Tools, and Quality Attributes
Recent advances in pharmaceutical sciences have led to nanoparticle-based drug delivery systems, enhancing the efficacy, safety, and bioavailability of drugs. This article reviews methods for developing these nanoscale dosage forms, emphasizing key techniques and notable studies.
1. Introduction to Nanoparticle-Based Dosage Forms
Nanoparticles, small carriers for drugs, improve delivery to specific body sites. Their high surface area and ability to navigate biological barriers make them ideal for this purpose. Developing these dosage forms involves various methods tailored to the drug's characteristics and desired outcomes.
2. Methods of Nanoparticle Synthesis
2.1 Emulsion-Based Techniques
Emulsion-based techniques, like single and double emulsion methods, are common for synthesizing nanoparticles. These involve dispersing a drug in an oily phase, emulsifying it in an aqueous phase with surfactants, then evaporating or extracting the solvent to yield controlled-size nanoparticles. Zhang et al. (2020) efficiently used a double emulsion method to produce PLGA nanoparticles for sustained drug release (Nature Reviews Drug Discovery, 2020).
2.2 Constituents of Emulsion-Based Techniques
Excipients are essential in emulsion-based nanoparticle formulation. They stabilize the emulsion, enhance drug solubility, and improve nanoparticle performance. Key excipients include:
2.2.1 Surfactants
Surfactants stabilize emulsions by lowering the interfacial tension between oil and water phases, aiding in the formation of stable droplets. Common surfactants used in emulsion-based nanoparticle formulations include:
· Polysorbates (Tween 20, Tween 80)
· Sodium dodecyl sulfate (SDS)
· Lecithin
· Span 60
2.2.2 Polymers
Polymers form the matrix of nanoparticles, encapsulating drugs and controlling release. Common polymers include:
· Poly(lactic-co-glycolic acid) (PLGA)
· Polycaprolactone (PCL)
· Chitosan
· Alginate
2.2.4 Oils
Oils constitute the internal phase of oil-in-water (O/W) emulsions or the external phase of water-in-oil (W/O) emulsions. Examples include:
· Triglycerides
· Mineral oils
· Essential oils
2.2.5 Co-Surfactants
Co-surfactants stabilize emulsions and enhance drug solubilization. Common examples include:
· Ethanol
· Polyethylene glycol (PEG)
· Propylene glycol
3. Tools for Emulsion-Based Nanoparticle Formulation
Formulating nanoparticles with emulsion-based techniques requires specialized tools to precisely control their size and distribution, ensuring optimal performance.
3.1 High-Shear Mixers
High-shear mixers create fine emulsions by applying intense mechanical energy, reducing droplet size and forming smaller nanoparticles.
3.2 Ultrasonicators
Ultrasonicators employ ultrasonic waves to generate cavitation bubbles, facilitating the breakdown of droplets into nanoparticles. This technique is highly effective in achieving a uniform size distribution.
3.3 Microfluidizers
Microfluidizers utilize high-pressure narrow channels to produce fine emulsions and uniform nanoparticles. They are essential for scaling up the production of nanoparticle formulations.
3.4 Homogenizers
Homogenizers increase pressure on emulsions to make droplets smaller, improving nanoparticle dispersion stability and uniformity.
4. Critical Quality Attributes (CQA)
Critical Quality Attributes (CQA) are properties that must be controlled to ensure the quality of nanoparticle dosage forms. Key CQAs for emulsion-based nanoparticles include:
4.1 Particle Size and Size Distribution
Nanoparticle size and distribution are vital for stability, bioavailability, and drug release. Techniques like dynamic light scattering (DLS) and laser diffraction measure these parameters.
Common tools to measure particle size distribution include:
1. Dynamic Light Scattering (DLS): This method uses laser light scattering by suspended particles to measure size distribution, ideal for nanoparticles and proteins. Common instruments include the Zetasizer Nano Series (Malvern Panalytical).
2. Laser Diffraction: Laser diffraction determines particle size distribution by capturing the light pattern scattered by particles under a laser beam. It measures sizes from submicron to millimeters. One example is Malvern Panalytical's Mastersizer.
3. Nanoparticle Tracking Analysis (NTA): NTA observes and measures the Brownian motion of nanoparticles to determine their size individually. The NanoSight by Malvern Panalytical is a well-known instrument for this method.
4. Transmission Electron Microscopy (TEM): TEM offers high-resolution images of nanoparticles, enabling the direct measurement of their size and morphology. Although it is not commonly used for routine measurements due to its complexity and cost, TEM is useful for detailed analysis.
5. Scanning Electron Microscopy (SEM): SEM provides detailed imaging of particles, albeit with somewhat lower resolution compared to TEM. It is particularly effective for examining the surface morphology and size distribution of nanoparticles.
6. Atomic Force Microscopy (AFM): AFM scans a probe over a sample's surface for nanoscale topographical imaging, measuring particle size and shape.
4.2 Encapsulation Efficiency
Encapsulation efficiency measures the percentage of a drug encapsulated in nanoparticles compared to the initial amount used. High encapsulation efficiency is essential for optimal therapeutic effects.
Encapsulation efficiency is a critical parameter in the pharmaceutical industry for ensuring that the correct dosage of a drug is delivered with nanoparticle formulations.
Several methods and tools are commonly employed to measure encapsulation efficiency:
1. Ultracentrifugation: This method separates nanoparticles from the free drug by centrifugation at high speeds. After separation, the concentration of the free drug in the supernatant is measured using spectrophotometry or high-performance liquid chromatography (HPLC). The encapsulation efficiency is then calculated by comparing the amount of the encapsulated drug to the total amount used.
2. Dialysis: Dialysis involves placing the nanoparticle suspension in a dialysis bag, which is then immersed in a suitable buffer. The free drug diffuses out of the bag, while the encapsulated drug remains inside. The concentration of the free drug in the dialysate is measured, and the encapsulation efficiency is determined accordingly.
3. Gel Permeation Chromatography (GPC): GPC separates nanoparticles from free drug molecules based on their size. By analyzing the elution profile and comparing the areas under the curve, the encapsulation efficiency can be quantitatively assessed.
4. Extraction and Quantification: This method involves disrupting the nanoparticles to release the encapsulated drug, which is then quantified using techniques like HPLC or UV-Vis spectrophotometry. This direct approach provides an accurate measure of the encapsulated amount.
5. Fluorescence Spectroscopy: For drugs that have intrinsic fluorescence or are tagged with a fluorescent marker, fluorescence spectroscopy can be used to determine the encapsulation efficiency. The fluorescence intensity of the encapsulated drug is compared with that of the total drug to calculate the efficiency.
4.3 Zeta Potential
Zeta potential measures nanoparticle surface charge, influencing stability and interaction with biological membranes. High absolute zeta potential values indicate good stability. Zeta potential is an essential parameter that reflects the surface charge of nanoparticles, significantly impacting their stability and interactions with biological membranes. High absolute values of zeta potential generally indicate enhanced stability due to electrostatic repulsion, which prevents aggregation. Therefore, precise measurement of zeta potential is crucial for ensuring the quality and efficacy of nanoparticle-based dosage forms.
· Zeta potential analyzer: Instruments such as the Zetasizer Nano Series (Malvern Panalytical) are commonly used for zeta potential measurement.
· Sample cells or cuvettes: Specialized cells designed for zeta potential analysis.
· Dispersion medium: A suitable medium (e.g., water, buffer) to disperse the nanoparticles.
· Nanoparticle sample: The nanoparticle-based dosage form to be analyzed.
· Standard solutions: Calibration standards for verifying the accuracy of the instrument.
· Avoiding Bubbles: Carefully handle the sample to avoid the introduction of air bubbles that can interfere with the measurement.
4.4 Drug Release Profile
The drug release profile details the rate and extent of drug release from nanoparticles, crucial for sustained and controlled delivery.
Several analytical tools and methods are used to determine the drug release profile of nanoparticle-based drug delivery systems. These tools help in understanding the release kinetics, which are critical for ensuring effective and sustained drug delivery.
Some of the commonly used tools include:
1. Dissolution Test Apparatus: Apparatus such as USP Type I (basket) and Type II (paddle) dissolution testers are widely used to simulate various physiological conditions and measure the rate of drug release from nanoparticles.
2. Dialysis Method: This involves the use of dialysis membranes to separate nanoparticles from the release medium, allowing the measurement of drug concentration over time.
3. High-Performance Liquid Chromatography (HPLC): HPLC is extensively used for quantifying the drug concentration in the release medium at different time intervals, providing precise and accurate release profiles.
4. Ultracentrifugation: This technique is used to separate the nanoparticles from the release media quickly, enabling the collection of samples for drug concentration analysis.
5. Spectroscopic Methods: UV-Vis spectroscopy and fluorescence spectroscopy can be used to monitor the drug release by measuring changes in absorbance or fluorescence over time.
6. In vitro Cell Culture Models: These models simulate the biological environment and can provide valuable information about the drug release and subsequent cellular uptake.
7. Mathematical Modeling: Software tools and mathematical equations are employed to simulate and predict the drug release kinetics based on experimental data.
4.5 Stability
Stability assessments evaluate nanoparticles' physical and chemical stability by monitoring changes in size, drug content, and morphology over time.
The FDA has approved several methods for studying the stability profile of nanotechnology in pharmaceutical dosage forms. Stability assessments are crucial in ensuring that nanoparticles maintain their physical and chemical integrity over time.
Here are some of the recognized methods:
1. Dynamic Light Scattering (DLS): This method measures nanoparticle size distribution and stability in suspension, detecting changes that may indicate aggregation or degradation.
2. Zeta Potential Measurement: Zeta potential indicates the surface charge and stability of nanoparticles, helping predict their long-term stability.
3. Transmission Electron Microscopy (TEM): TEM offers detailed images of nanoparticles, enabling the observation of changes in morphology and structure over time, which are essential for stability assessments.
4. Differential Scanning Calorimetry (DSC): DSC measures the thermal properties of nanoparticles, such as melting point and glass transition temperature. These properties can indicate the stability of the drug encapsulated within the nanoparticles.
5. High-Performance Liquid Chromatography (HPLC): HPLC is used to quantify the drug content within nanoparticles and detect any degradation products over time, ensuring the chemical stability of the formulation.
6. UV-Vis Spectroscopy: This method can monitor the absorption spectra of nanoparticles to detect any changes in their optical properties, which may be indicative of instability.
References:
- Nanotechnology: Principles and Practices by Sulabha K. Kulkarni (Springer)
- Particle Size Analysis in Industrial Hygiene by John M. Conner (Van Nostrand Reinhold)
- "Characterization of Nanoparticles-Based Drug Delivery Systems" in the journal Drug Delivery and Translational Research.
- "Techniques for the Characterization of Nanoparticles in Pharmaceutical Formulations" in The AAPS Journal.
- "Quantitative Analysis of Encapsulation Efficiency in Nanoparticle Drug Delivery Systems" in Pharmaceutical Research.
- Nanotechnology: Principles and Practices by Sulabha K. Kulkarni (Springer)
- Particle Size Analysis in Industrial Hygiene by John M. Conner (Van Nostrand Reinhold)
- "Characterization of Nanoparticles-Based Drug Delivery Systems" in the journal Drug Delivery and Translational Research.
- "Techniques for the Characterization of Nanoparticles in Pharmaceutical Formulations" in The AAPS Journal.
- "Quantitative Analysis of Encapsulation Efficiency in Nanoparticle Drug Delivery Systems" in Pharmaceutical Research.















Comments